Initiating High-Dose Oral Testosterone
Undecanoate Therapy in Hypogonadal Men
Jimmy Vo BS, Grace Yoon BS, and Andrew Y. Sun MD
Urology Partners of North Texas, Arlington, Texas, USA
Introduction
- Oral testosterone undecanoate is a novel form of testosterone replacement therapy.
- Oral TU is conventionally initiated at 200mg BID (Kyzatrex), 237mg BID (Jatenzo), or 225mg BID (Tlando).
- Many patients attain greater symptomatic benefit at higher doses.
- In our practice we often initiate therapy at a starting dose of 400mg BID.
Results
- 27 patients met inclusion criteria and had complete data
- Mean follow up time = 6 months
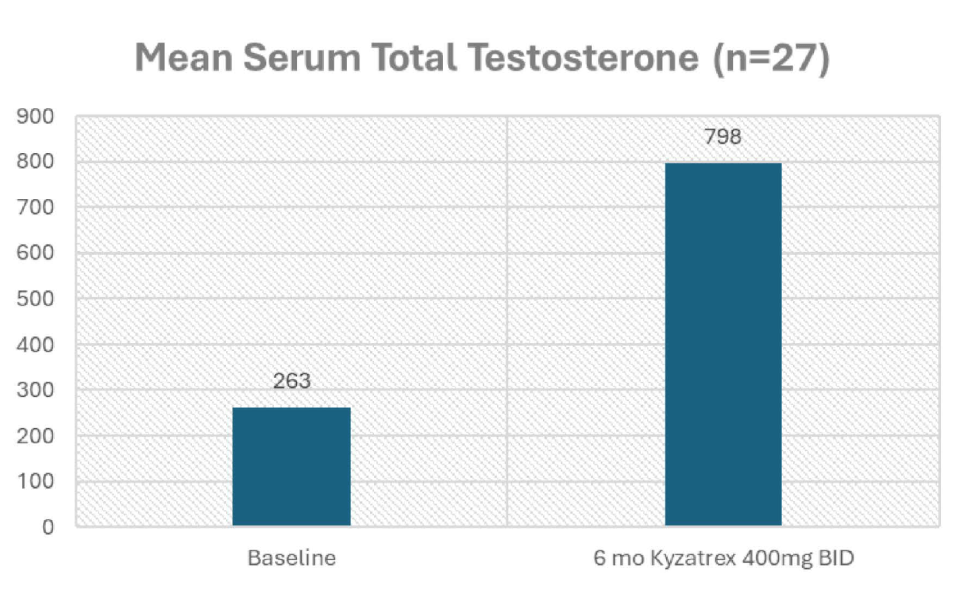
- Significant increase in TT (263 to 798 ng/dL).
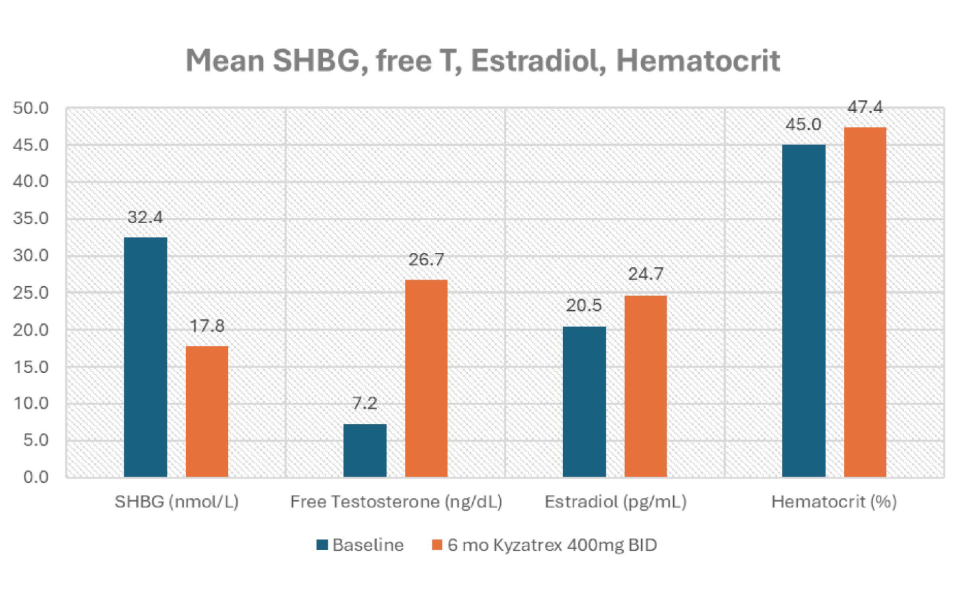
- Drop in SHBG (32.4 to 17.83 nmol/L).
- Increase in calculated fT (7.24 to 26.74 ng/dL).
- Estradiol modestly increased (20.5 to 24.7 pg/ml).
- Hematocrit did not significantly increase (44.9% to 47.4%).
Aim
- Retrospectively report the outcomes of starting hypogonadal men on oral TU at the maximum available dose of 400 mg BID
- Safety
- Efficacy
- Patient satisfaction
- Adherence
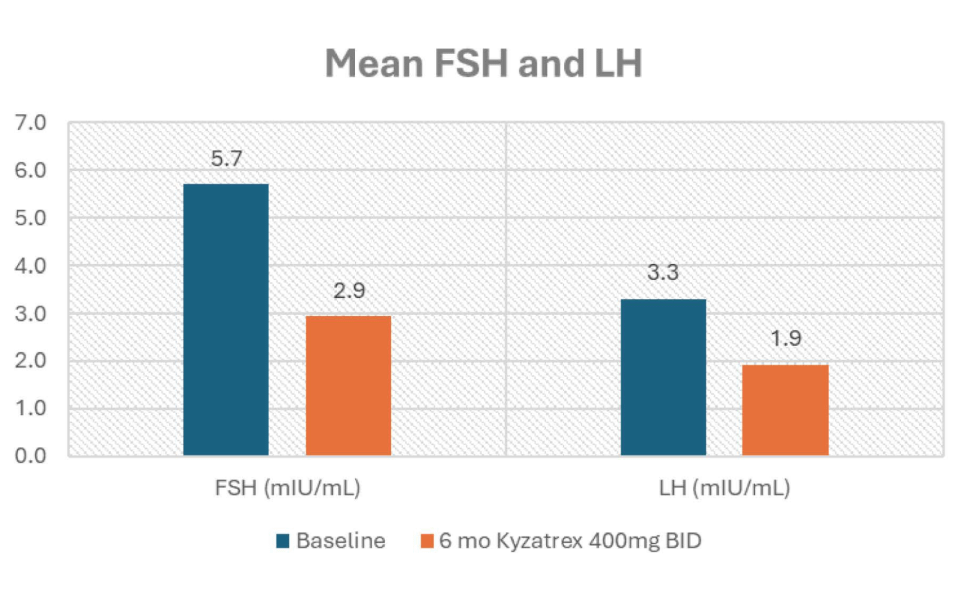
- FSH and LH were maintained at non-zero levels: (FSH from 5.7 to 2.9 mlU/mL and LH from 3.3 to 1.9 mlU/mL).
Method
- Retrospective, single-center chart review
- Hypogonadal men treated with a starting dose of oral TU at 400mg BID from August 2023 to April 2024.
- Satisfaction was subjectively recorded
- Adherence was measured by prescription refill records.
- Total testosterone (TT)
- Sex Hormone Binding Globulin (SHBG)
- Calculated Free Testosterone (fT)
- Estradiol (E2)
- Hematocrit (Hct)
- Follicle stimulating hormone (FSH)
- Luteinizing hormone (LH).
- No patients reported testicular atrophy.
- No patients were initiated on aromatase inhibitors.
- One patient had a hematocrit rise above 52% (53.2%) and was reduced to 300mg BID with resolution of his erythrocytosis.
- Side effects were rare with 2 patients (7.4°/o) reporting transient GI upset.
- Subjective patient satisfaction was high, with 26/27 (96%) of patients reporting improvement in symptoms and continuing therapy.
Conclusion
- Initiating oral TU therapy with Kyzatrex at 400mg BID is safe and effective.
- The high dose was well-tolerated and resulted in substantial symptom improvement, high patient satisfaction, and adherence.
- These findings support considering a higher starting dose for hypogonadal men considering oral TU therapy.
Combined Clomiphene and High-Dose Oral Testosterone
Therapy in Hypogonadal Men: A Case Series
Grace Yoon BS, Jimmy Vo BS, and Andrew Y. Sun MD
Urology Partners of North Texas, Arlington, Texas, USA
Introduction
- Clomiphene is often used in the treatment of hypogonadal men who wish to preserve endogenous testosterone production
- However symptomatic responses are often suboptimal.
- Exogenous testosterone replacement, while effective, typically suppresses the hypothalamicpituitary-gonadal (HPG) axis.
Results
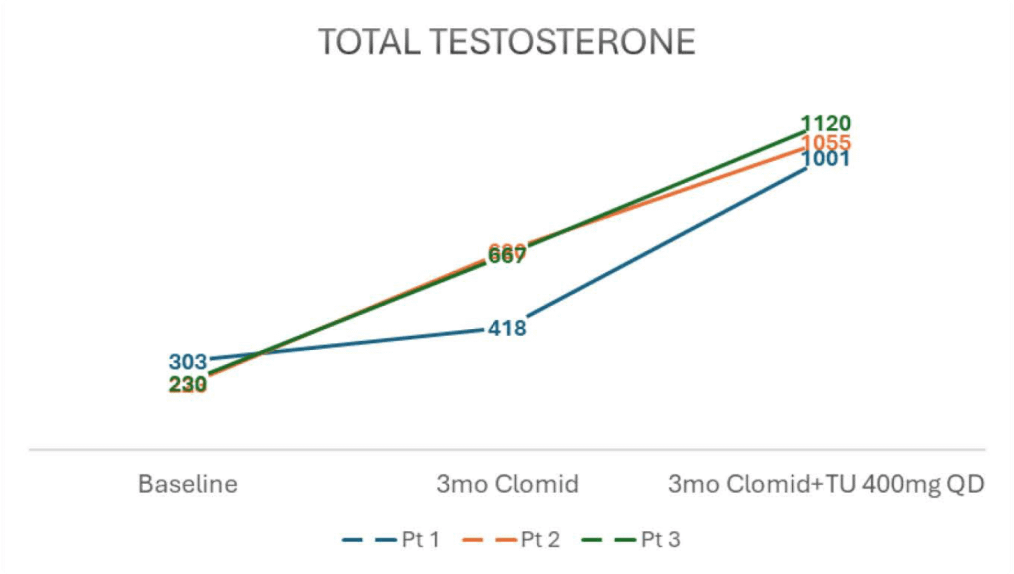
- Baseline serum testosterone levels of 303, 223, and 230 ng/dl.
- After 3 months of clomiphene 50mg QoD, TT rose to 418, 680, and 667 ng/dl
- Add 400mg oral TU QD for 3 months, TT rose to 1001, 1055, and 1120 ng/dl
- Patients reported significant improvement in symptoms such as erectile dysfunction, fatigue, libido, and exercise tolerance.
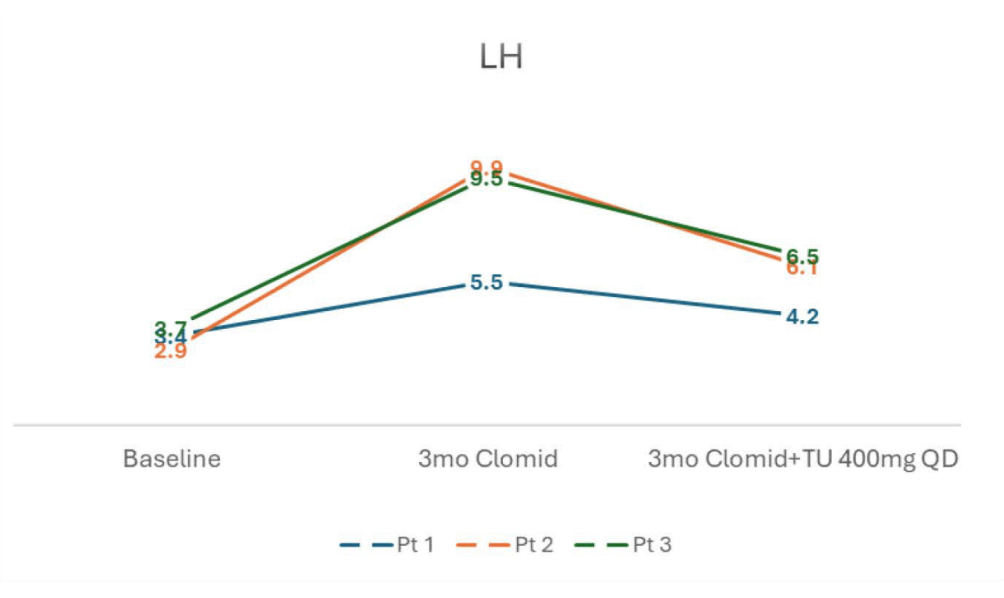
- FSH and LH levels both rose on clomiphene and subsequently dropped after initiation of oral TU, but remained near baseline
Aim
- This case series investigates the efficacy of combining clomiphene with high dose oral testosterone undecanoate (TU) (Kyzatrex ®, Marius Pharmaceuticals).
- 3 Patient case series
Method
Three hypogonadal men:
- Initially received clomiphene therapy (50mg QoD) with poor symptomatic improvement
- Given oral TU 400mg once daily with lunch.
- Evaluated after 3 months of clomiphene monotherapy
- Evaluated again after 3 additional months of clomiphene + Oral TU combination therapy.
- Total testosterone (TT)
- Sex Hormone Binding Globulin (SHBG)
- Calculated Free Testosterone (fT)
- Estradiol (E2)
- Hematocrit (Hct)
- Follicle stimulating hormone (FSH)
- Luteinizing hormone (LH).
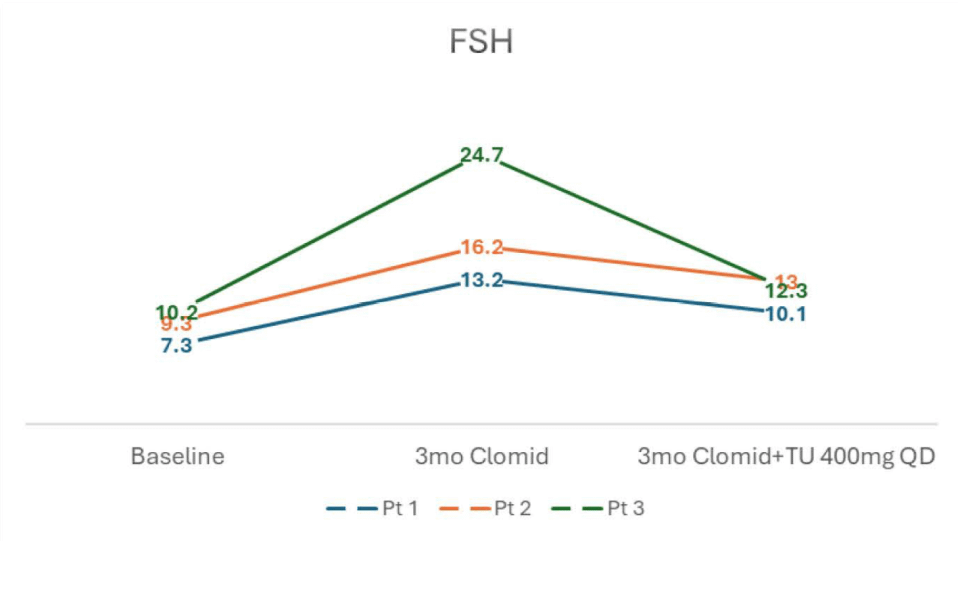
- SHBG and Estradiol levels rose with clomiphene monotherapy but subsequently decreased after adding oral TU.
- Hct was unchanged throughout.
- No significant adverse events were reported.
Conclusion
- SHBG and Estradiol levels rose with clomiphene monotherapy but subsequently decreased after adding oral TU.
- Hct was unchanged throughout.
Effects of Oral Testosterone Undecanoate on Semen Parameters in
Hypogonadal Men: An Interim Analysis of a Prospective Pilot Study
Gal Saffati, Daniela Orozco Rendon, Shane Kronstedt, David E. Hinojosa-Gonzalez, Mohit Khera.
Baylor College of Medicine, Houston, TX
Introduction
- Testosterone replacement therapy (TRT), per the AUA guidelines, is indicated for men with hypogonadism who do not want to preserve fertility.
- While injectable testosterone preparations are widely used, oral testosterone formulations have become an increasingly popular treatment option due to their ease of administration and avoidance of repeated intramuscular injections.
- However, the effects of oral testosterone on semen analysis (SA) parameters and male fertility potential remain understudied.
Results
Table 1. Baseline and follow-up values

T: testosterone; FSH: follicle stimulating hormone, LH: luteinizing hormone; SA: semen analysis
Aim
- To evaluate changes in SA parameters, among hypogonadal men receiving oral testosterone undecanoate therapy.
Method
- Design: A single-arm pilot prospective study, unblinded.
- Inclusion:
- 18-49 years old who qualify for TRT.
- TRT naïve.
- Exclusion:
- Prior TRT.
- Prior use of clomiphene citrate or hCG.
- Demonstrated azoospermia.

Conclusion
- Despite a substantial increase in total and free testosterone levels after 3 months of therapy, the overall semen analysis parameters—sperm concentration, motility, and volume—remained largely unchanged.
- The exception of one patient developing azoospermia underscores the potential impact of testosterone therapy on fertility in certain individuals, though this was not a widespread effect in the cohort.
- While these early results suggest that oral testosterone undecanoate may not universally impair semen parameters in the short term, we highlight the need for larger and longer-term studies to fully elucidate the potential reproductive risks associated with this therapy

