Actor Portrayal
Simplifying Testosterone Treatment
KYZATREX®:
The Next Level in
Testosterone
Replacement Therapy
Offer patients a simple, painless way to manage low testosterone with twice-daily oral capsules.
KYZATREX is helping patients ditch the painful injections and messy gels for an oral option that fits effortlessly into everyday life.

*In a six-month open-label clinical study (n=139) of men with low testosterone, 88% achieved Cavg 222-800 ng/dL at Day 90 (worst case scenario calculation, excluding Site 104, 95% CI, 82% to 93%). Based on end-of-study completers (n=127), 96% of patients achieved Cavg 222-800 ng/dL at Day 90 (95% CI, 93 to 99%).
An Oral Formulation of
Testosterone Undecanoate
KYZATREX is an oral formulation of testosterone undecanoate (TU) designed to utilize lymphatic absorption to bypass first-pass metabolism and drive uptake. Utilizing a self-emulsifying drug delivery system (SEDDS) incorporating phytosterol esters, KYZATREX was designed for clinical efficacy and safety.¹
Lymphatic Absorption
KYZATREX is well absorbed from the GI tract when taken with food.
PK Profile1
KYZATREX is an immediate-release formulation with twice-daily dosing that, when administered every 12 hours, closely mimics the diurnal rhythm of endogenous testosterone.
Mean Concentration-Time Profile (N=139)
Mean (Standard Error of the Mean) Testosterone Concentration (ng/dL) Post
Morning KYZATREX Dose at Day 90
Proven to Boost Testosterone
The efficacy of KYZATREX was evaluated in a 6-month open-label clinical study involving 139 hypogonadal men (median age of 50 years; range 22 to 66 years). The primary efficacy endpoint was the percentage of KYZATREX-treated patients with mean plasma total testosterone concentration (Cavg) over 24-hours within the normal range of 222-800 ng/dl on the final PK visit of the study at Day 90. KYZATREX demonstrated the following at Day 90:
*Sex Hormone Binding Globulin (SHBG) binds to testosterone and decreases free testosterone levels. The less SHBG you have, the higher your free testosterone levels will be.2
Demonstrated Safety Profile
Endpoint
MRS-TU-2019EXT Study (N = 155)
ABPM
Change in 24-hour mean SBP from baseline after 4 months of KYZATREX, mmHg (95% CI)
1.7 (0.3-3.1)
Change in 24-hour mean SBP from baseline after 6 months of KYZATREX, mmHg (95% CI)
1.8 (0.3-3.2)
In-clinic Blood Pressure Cuff
Change in 24-hour mean SBP from baseline after 4 months of KYZATREX, mmHg (95% CI)
0.2 (0.9-4.5)
Change in 24-hour mean SBP from baseline after 6 months of KYZATREX, mmHg (95% CI)
2.4 (0.6-4.2)
(Mean ∆ from baseline at 6 months)
PSA
 0.15 (±0.04) ng/mL
0.15 (±0.04) ng/mL
Hemoglobin
 0.48 g/dL
0.48 g/dLCholesterol
 11.1 mg/dL
11.1 mg/dL
Triglycerides
 18.6 mg/dL
18.6 mg/dL
Fasting Insulin†
 5.2 μU/mL
5.2 μU/mL
†Data from MRS-TU-2019 (n=162) at 12 months. MRS-TU-2019 had a different dosing scheme than MRS-TU-2019EXT.
ABPM: ambulatory blood pressure monitoring; CI: confidence interval; SBP: systolic blood pressure.
Adverse Events in ≥2% of Patients Receiving KYZATREX1
Hypertension: 2.6%
One patient discontinued because of an adverse reaction (acne).
No patients discontinued due to erythrocytosis1.
In a 12-month, open-label clinical study, men who received KYZATREX 200 mg QD to 400 mg BID (n = 202) reported the following additional adverse reactions: headache, arthralgia, diarrhea, hemoglobin increased, anxiety, constipation, peripheral edema, and prostate-specific antigen increased.1
Patient Reported Outcomes
In a clinical study, KYZATREX patients (n=214) reported the following improvements as a result of improved testosterone levels (compared to baseline):2
Exploratory endpoints from Study MRS-TU-2019; Different dosing scheme than MRS-TU-2019EXT.
Results may not be clinically significant.
- Overall Level of Sexual Desire
- Positive Mood
- Sexual Activity Score
- Erection Grade
- Erection Duration
- Fatigue
- Energy
- General Health
- Social Functioning
- Erectile Function
- Intercourse Satisfaction
- Orgasmic Function
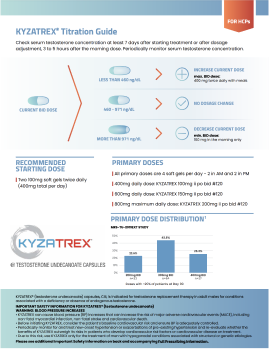
Daily Oral Dosing
Testosterone-boosting benefits in two tiny capsules taken twice daily
KYZATREX is not a gel, injection, pellet, or patch. Just two KYZATREX oral doses a day help restore testosterone to normal levels and keep it there. KYZATREX should be taken with food, but no dietary modifications are required.
Flexible Dosing
Meet your patient’s testosterone needs with a range of KYZATREX doses.

References:
- KYZATREX [prescribing information]. Raleigh, NC: Marius Pharmaceuticals; 2023.
- Data on file. Raleigh, NC: Marius Pharmaceuticals, 2020.